In this course you will:
Identify how to overcome the operational and clinical barriers that may interfere with optimal biomarker-based testing
Identify the appropriate biomarker test based on type of tissue sample
Identify available options to address gaps and obstacles to biomarker testing
Utilize biomarker testing results to inform treatment decisions
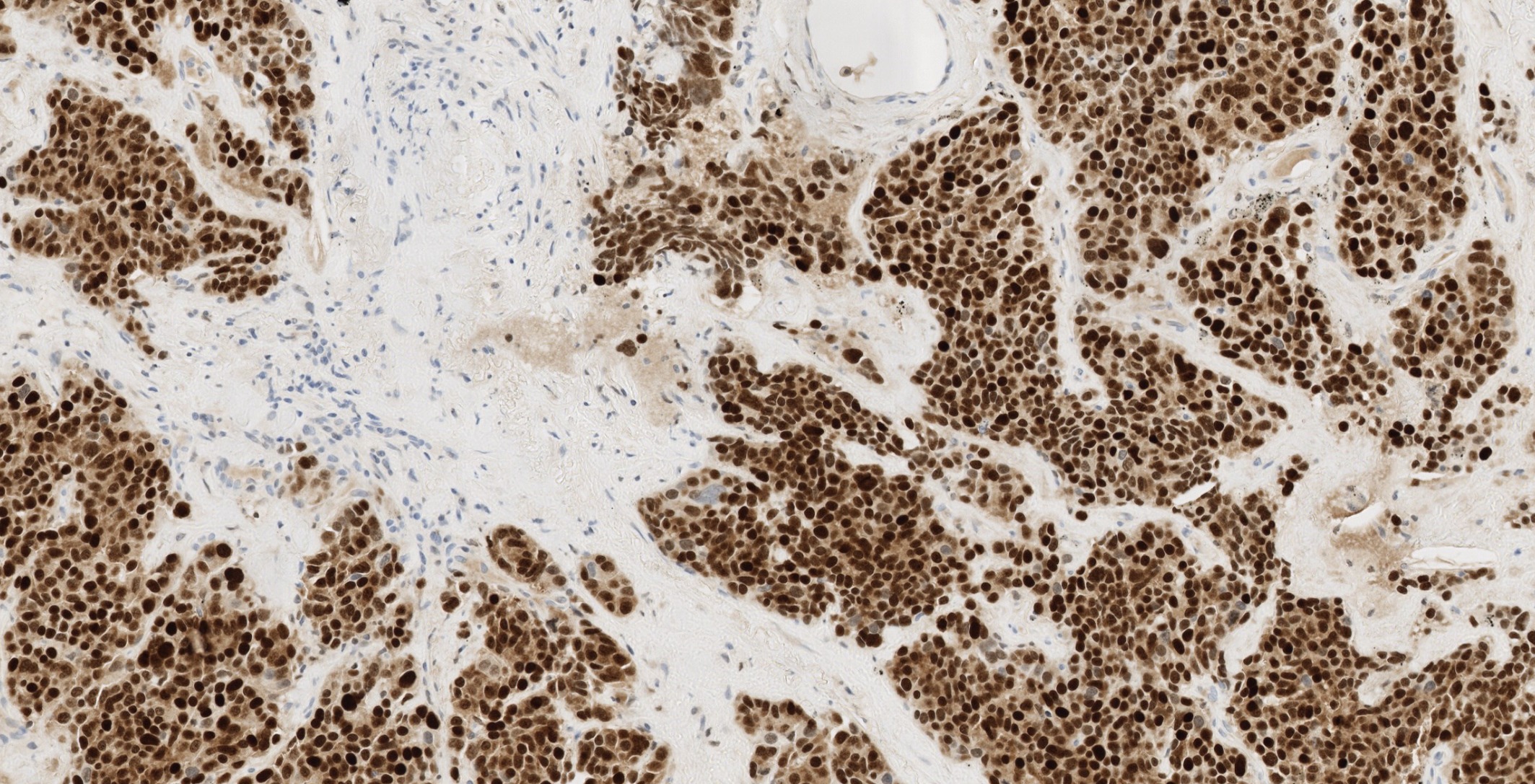
Digital slide box showing 11 cases (SCLC, normal pancreas, carcinoid, NSCLC) stained with VENTANA INSM1 (SP493) Rabbit Monoclonal Primary Antibody, with annotations for H&E, Negative Reagent Control, and immunohistochemistry stained slides.
This series of videos is provided as a resource for individuals in countries where VENTANA PD-L1 (SP263) Assay is approved for use for non-small cell lung cancer (NSCLC).
Please refer to the respective drug labeling for clinical recommendations pertaining to PD-L1 expression.
These videos feature Roche Tissue Diagnostics Pathologist, Dr. Dorothy Hayden, and are intended to be a refresher for pathologists and laboratories on VENTANA PD-L1 (SP263) Assay in NSCLC. Module 1 is a PD-L1 and PD-L1 (SP263) assay overview , Module 2 discusses evaluation guidance for PD-L1 (SP263) assay in NSCLC, and Module 3 is a PD-L1 (SP263) Guided Digital NSCLC Case Review
Virtual Training on assessing PD-L1 in non-small cell lung cancer using the VENTANA PD-L1 (SP142) assay.
Virtual Training on assessing PD-L1 in non-small cell lung cancer using the VENTANA PD-L1 (SP263) assay.